Enterovirus 71 Causes Mouth Foot Disease
Hand - Foot - Mouth is a dangerous infectious disease that is common in young children. Signs of the disease of the mouthpiece in children are easily recognizable as a high fever does not lower the fever; skin lesions such as burnout, bruises in the throat area, around your mouth, palms, feet, ass, knees, etc. Especially cases of children abandoning food, vomiting, increased saliva, diarrhea, fatigue, and disturbance crying.
The main causes are Coxsackievirus A16 (group A16) and Enterovirus 71. (EV71). In mild-symptomatic cases commonly known as group A16 viruses, the remaining cases identified with enterovirus 71 will usually be severe, potentially dangerous complications.
The prevention of epidemics in our country has improved much more with many widespread prevention measures. Recently, however, the disease of the mouth hand has begun to show signs of reappearance in the southern region of our country.
According to a report from the City Centers for Disease Control earlier this year, severe cases began to rise in combination with the incidence of Enterovirus 71 detected by PCR testing.
Because there are no specific vaccines for the disease of the mouth legs, treatment options along with the programmed medications have been given to improve the condition of the infected subject. However, prevention rather than cure, the Department of Health is considering approving an oral hand-and-foot vaccine, capable of preventing the EV71 virus, the most dangerous strain, that can be injected in the years to come.
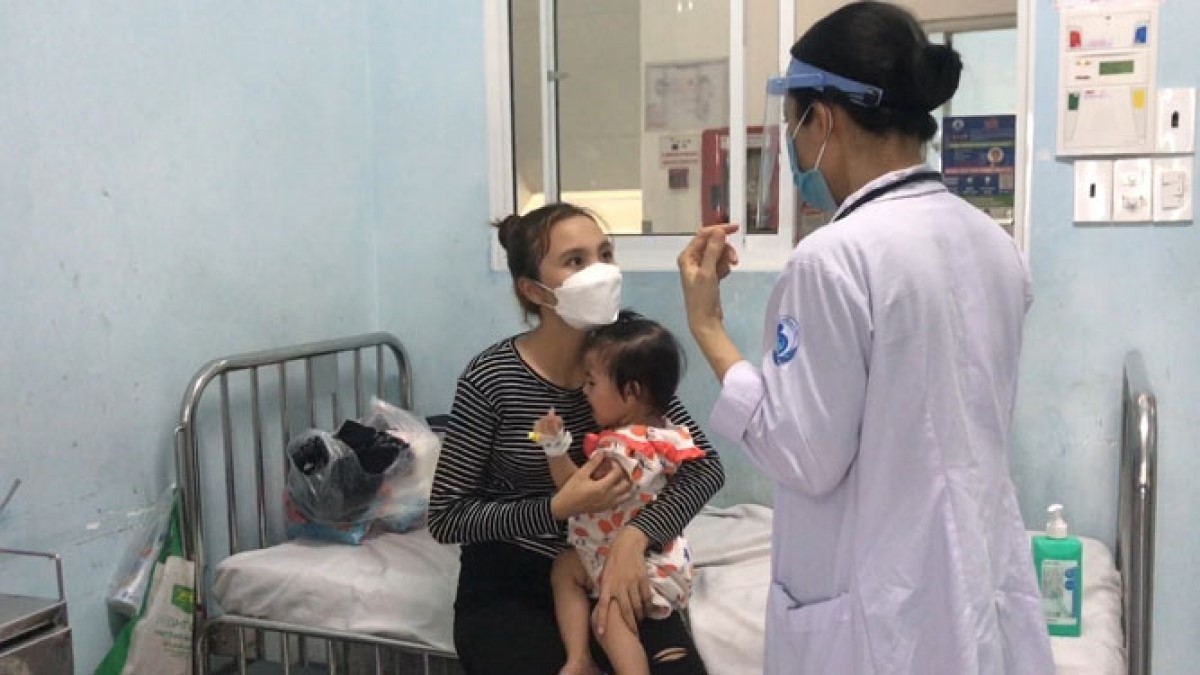
Enterovirus 71 Causes Mouth Foot Disease
How do you test the EV71 vaccine?
The discovery and testing of vaccines against the disease is a very joyful signal to our country because there is still no specific treatment for the disease. The Ministry of Health has authorized the registration of phase three clinical trials in Vietnam with the research coordinator, the Pasteur Institute in Ho Chi Minh City.
Research Phase 1, 2
The EV71 vaccine was first studied by the National Health Research Institutes (NHRI), Taiwan. After the first phase of the study, the vaccine was transferred to develop subsequent phases of clinical trials.
The vaccine is an inactive vaccine that prevents the disease caused by the EV71 virus. In 2010, up to 60 volunteers were given immune birth results and were completely healthy after completing phase 1 clinical trials.
Until Phase 2, the clinical trial continued with 400 participants aged between 2 months and 12 years. The majority gave positive results, even the elderly half of subjects receiving the vaccine increased the NTAb value by four times or more after the injection (the level of anti-virus-capable antibodies increased four times after injecting).
Phase of Study 3

Testing process of the EV71 vaccine in Vietnam
Up to Phase 3, this is the research phase in which Vietnam has the honor to participate in research along with testing points in Taiwan. Clinical trials at this stage are also key to evaluating the protective effectiveness of vaccines.
The southern part of our country is one of the hotspots of the epidemic, so it has been prioritized for actual clinical trials of EV71 vaccines. To date, two clinical trials have been conducted in Vietnam, one completed (2019-2021, the vaccine is being licensed) and one is underway. (2023 - 2025).
With the vaccine study completed, the results were published in The Lancet as a prestigious UK sanitary overview week. The study recruited more than 2,500 children in 6 districts in the two provinces of Tien Giang, Dong Thap of Vietnam.
With the vaccine study underway, the study focused on subjects from 2 months to under 6 years of age. The results showed that the study vaccine helped protect children against EV71 at any severity, with a protection rate of 96.8%.
In the third phase of the trial study, no cases of mouthfulness were in the group of children vaccinated during the two years of the study. Experimental studies also showed that the expected adverse events that occurred during the study were mostly mild and could recover on their own. There were no serious adverse events associated with the study vaccine.
When is EV71 vaccine available in Vietnam?
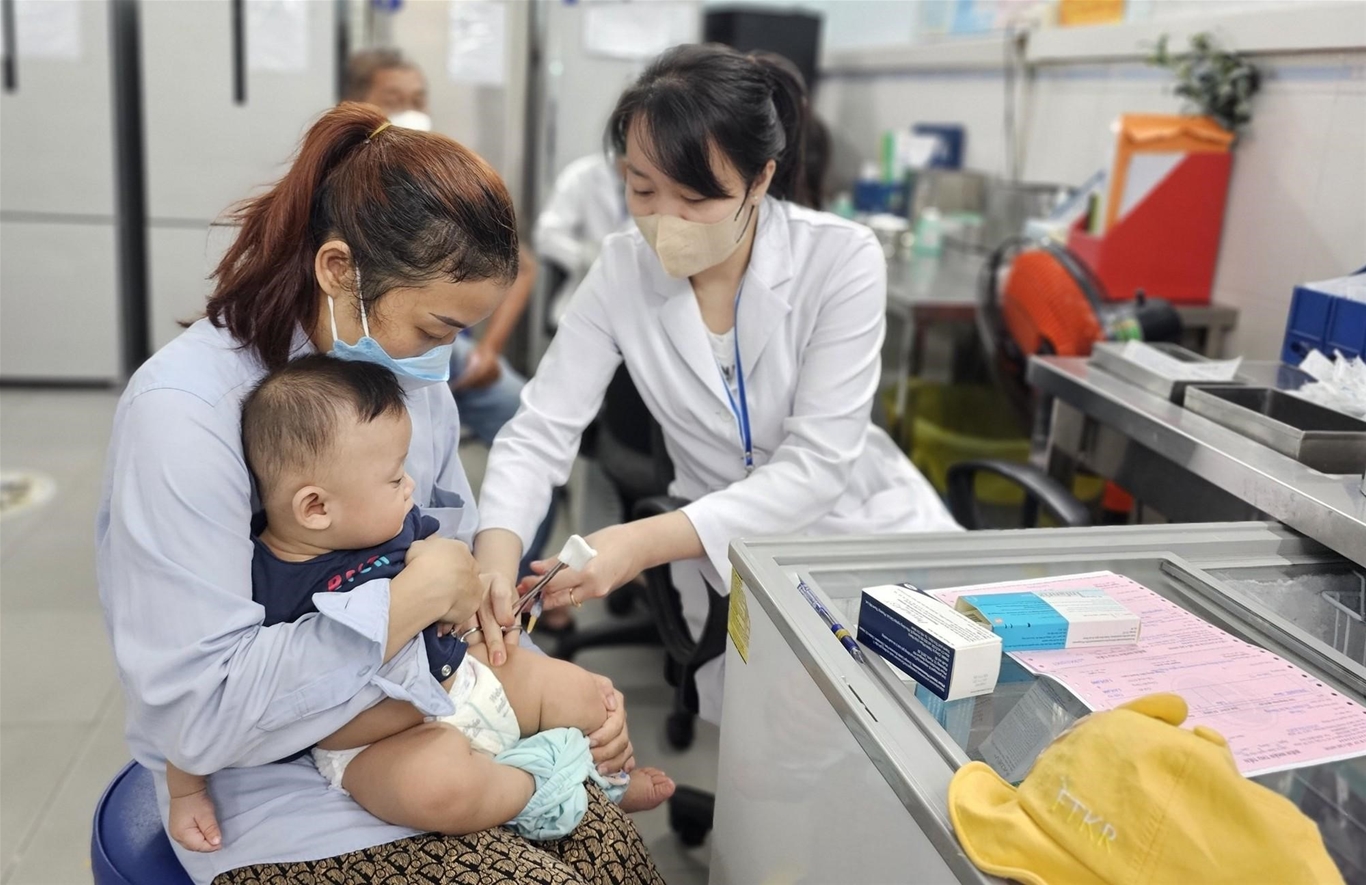
Vietnam will soon have an oral foot vaccine
According to the Administration of Pharmaceuticals (Ministry of Health), the vaccine is being licensed in Vietnam to actively immunize infants and young children from 2 months to 6 years of age to prevent EV71 infection. Therefore, more time is needed from now until the end of 2023, early 2024, for the vaccine to be approved before it can be placed on the market for injection services, contributing to reversing the epidemic.
If approved and marketed, it will be the first Hand - Foot - Mouth vaccine in Vietnam to use EV71 inactivation technology. With the method of breeding pathogens, usually viruses, in suitable environments, until they are fully developed, heat, chemicals or radiation will be used to destroy genetic material and "kill" these microbes. It is also a commonly used technology to produce vaccines with many properties that help protect the body from disease-causing agents, thus giving better vaccine test results.
VIETSTAR BIOMEDICAL RESEARCH is a research-supporting organization that provides a range of clinical trials and legal services for the development of pharmaceutical products and biotechnology from Phase I to Phase IV in Vietnam.
If you are reading this article and need support in the field of clinical trial research, do not hesitate to contact us as below!
VIETSTAR BIOMEDICAL RESEARCH
Office Address: Room 201, 2nd Floor, N01T1 Tower Office Building, Diplomatic Complex, Bac Tu Liem District, Hanoi, Vietnam
Tel/ Fax: + (84 4) 32 000 867 - Hotline: + 84 (0) 903 40 43 34, + 84 (0) 989 18 88 07
Email: contact@vietstar-research.com
Website: www.vietstar-research.com