Using modeling and simulation to optimize your chances of clinical success
Quantitative Clinical Development (QCD) combines clinical pharmacology modeling and simulation with clinical PK/PD and pharmacometrics for a more strategic approach to clinical development, reducing the time and cost of bringing drugs to the market. By leveraging mathematical models for a quantitative analysis of the relationship between drugs, disease and patients, modeling and simulation can predict a drug’s benefits and adverse effects in a patient population prior to conducting a clinical trial. Model-based Drug Development (MBDD) can be implemented at any phase to improve the efficiency of clinical development by providing quantitative justification for trial design, dose selection, and decisions during trial execution. In addition, use of quantitative clinical pharmacology throughout the lifecycle of a drug candidate supports high quality regulatory packages to help secure regulatory approval.
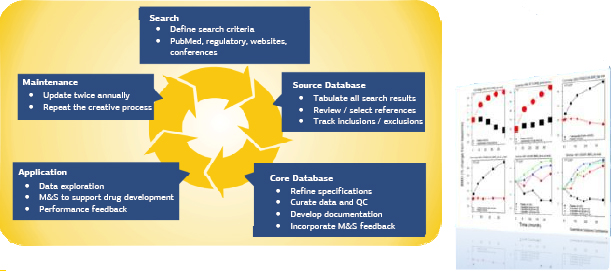
The path to smarter drug development is clear with VIETSTAR BIOMEDICAL RESEARCH’s modeling and simulation expertise
VIETSTAR BIOMEDICAL RESEARCH’s QCD group provides expertise in the strategic implementation of modeling & simulation services to help clients make smarter drug development decisions for a smoother journey to market.
Therapeutic Expertise Includes:
-
Oncology
-
Infectious Disease
-
Cardiology/Renal Neurology/Psychiatry
-
Metablolism/Endocrine
-
Respiratory
-
Immunology
-
Dermatology
-
Gastroenterlogy
-
Hematology
-
Hepatology
-
Rare Disease
Services include:
1. Quantitative Clinical Pharmacology
-
Strategic implementation of model-based development
-
Regulatory submissions & labeling
-
Biosimilar & Biobetter development strategies
-
Pediatric Development (PIP/PSP)
2. Pharmacometrics
-
PK/PD Analysis for efficacy & safety
-
Mechanistic modeling & simulation
-
Dose and dosing regimen selection
-
Disease & Placebo Models
-
Clinical Trial Simulation
3. Systems Pharmacology
-
Systems model development for selected targets
-
Identification of relevant biomarkers
-
Prediction of likelihood of achieving target profile
-
Integration of genetic markers to phenotype patient sub-populations
4. PK Analysis & Programming